|
In the 1980s, a number of pharmaceutical companies launched drug discovery efforts seeking to find drugs that would limit the secondary damage to brain tissue following acute traumatic brain injuries (TBI) or strokes, the latter including both ischemic and hemorrhagic brain insults. Most firms targeted the problem of TBI or stroke massive release of the excitatory neurotransmitter glutamate, exploring the efficacy of a number of glutamate receptor antagonists that might block the excitotoxic effects of excessive glutamate release.
In contrast, The Upjohn Company took a different neuroprotective approach based on the premise that it was more important to try to antagonize the neurodegenerative effects of the acute generation of highly reactive oxygen free radicals within the injured brain that could act as antioxidants and attenuate free radical induced lipid peroxidation, to which brain cell membranes had been shown to be highly sensitive.
Upjohn researchers discovered a promising group of 21-aminosteroid compounds that were highly effective neuroprotective antioxidants. It helped that Upjohn had world-leading steroid experience and expertise at the time. Some of these compounds were so effective in limiting brain damage in preclinical models of TBI, ischemic stroke, or subarachnoid hemorrhage (SAH) that the scientists involved in their discovery nicknamed the compounds “lazaroids” after the biblical character who rose from the dead. One of the lazaroids, tirilazad mesylate, appeared to be sufficiently protective across a range of preclinical neuroprotection paradigms that it might truly be worthy of the name lazaroids.
Tirilazad mesylate (product name - Freedox) was entered into an ambitious clinical development targeting moderate and severe TBI, thromboembolic stroke, and aneurysmal SAH beginning in early 1988, following approval of the Investigational New Drug Application by the U. S. Food and Drug Administration (FDA). A suitable intravenous formulation was developed and preclinical pharmacokinetic and toxicology studies were completed. These were followed by safety studies in uninjured human volunteers. Multicenter phase II and III clinical trials were initiated. The product name used was Freedox.
In the mid-1990s the FDA rejected a second application by Upjohn for approval of tirilazad mesylate/Freedox for the treatment of SAH. A member of the FDA advisory panel was quoted as saying, “I don't see efficacy in women or men, at this point”. One literature review concluded that the drug may have worsened stroke outcomes in women and that further trials of tirilazad were unwarranted. A second review concluded that the clinical evidence available was not strong enough to justify the routine use of tirilazad in the management of subarachnoid hemorrhage.
Sadly, this was the death knell for an extremely exciting development for the treatment of stroke. At this time, tirilazad mesylate was the only major candidate in the research pipeline at The Upjohn Company. Its failure left the company with no near-term new products. Projections for the future revenue stream of the company were grim.
Because of this situation, in 1995 The Upjohn Company board of directors were forced to look at options for purchase of or merging with another company. Few other companies were interested as The Upjohn Company was in bad shape but Pharmacia, a Swedish corporation that had no significant US presence, saw opportunity in a 50-50 merger. Most Upjohn employees in Kalamazoo had never even heard of Pharmacia. On August 21, 1995, news of a Upjohn-Pharmacia merger became public, the new company to be called Pharmacia & Upjohn.
Since then , neuroscience has undergone a striking change.
An ever increasing number of scientists and clinicians now recognize
that sex matters to brain function in a degree far greater and
more pervasive than had been understood in the 1990s. Some of them
believe it is time to revisit and kill the apparently erroneous
conclusion that tirilazad does not benefit any victims of stroke.
The evidence easily warrants, at minimum, that the effectiveness of
tirilazad for treating SAH in men should be revisited by all
relevant parties.
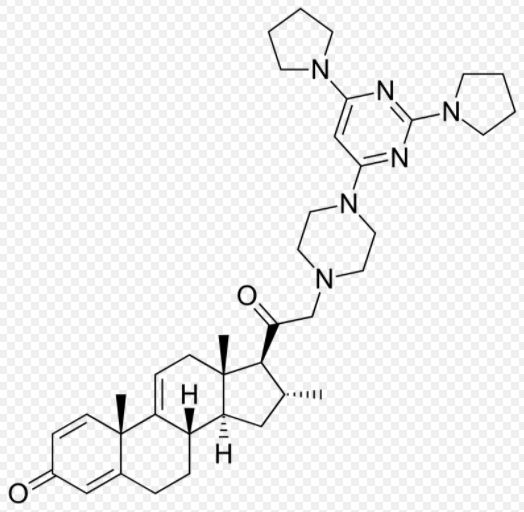
Molecular Formula for Tirilazad Mesylate
Written by Jeremy Winkworth, March 28, 2021
|